Interview with patient andreas wicki
«EVERYTHING HURTS»
A Patient’s Journey with Multiple Myeloma
Andreas Wicki’s journey is a tale of remarkable resilience. In his interview with Digest, his unwavering spirit shines through as the former FCZ sales manager narrates his battle with multiple myeloma. It becomes apparent that he is a fighter, refusing to be defeated. «In my life, nothing was handed to me on a silver platter. But the good thing that came out of it is that these challenges fueled my strength and opened my heart.»

Andreas Wicki informed his friends and followers on Twitter: «Since November 2018, my every- day life has been marked by many visits to doctors/hospitals. I have an incurable blood cancer. Multiple Myeloma!»
Photo courtesy of Andreas Wicki
Hailing from Wollerau, at the Lake of Zurich, Andy (49) was an optimistic individual with a zest for life. He always used to be on the move, playing soccer and tennis, engaging in weight training, and embracing every moment with enthusiasm. His wanderlust took him to various corners of the globe, and Despite his background in electrical installation, his passion for sports steered him towards a career as a sales manager for the football club FC Zürich.
However, the fall of 2018 marked a turning point in Andy’s life, an abrupt shift that would test his resilience to its core. At the age of 45, Andy found himself increasingly plagued by back pain. As November unfolded, the pain intensified, and Andy had a major breakdown while accompanying his then-girlfriend to the airport.
Back Pain is an Indication
On the drive home, the pain in his back became excruciating. It was so intense that Andy struggled to sit upright in his car. He got home to his friend waiting for his return, and together, they tried everything to get Andy out of the car, but their efforts proved futile.
After two hours of desperate struggle, they had no choice but to call for an ambulance. As the piercing wail of the ambulance siren shattered the silence, they were unaware that it signaled the beginning of a new chapter in Andy’s journey, one that demanded unwavering determination and the resilience of a true fighter.
The Shocking Discovery
The hospital’s cold, sterile walls were witnesses toAndy’s agony as the MRI scan revealed that something was wrong with his twelfth thoracic vertebra. The excruciating back pain persisted, with painkillers proving futile in their attempts to help Andy feel better.
«When the nursing staff rolled me over on the bed, I saw black. The pain was indescribable. I thought I was dying.»
Yet unbeknownst to Andy, the degenerated plasma cells in the bone marrow had softened his thoracic vertebra so much that it broke. When the doctors delivered this unfortunate news, a storm of emotions raged within Andy. He looked out of the window, a tear rolling down his face. His first question was, «What can I do?».
Soon Andy underwent surgery aimed at mending his fractured vertebra, and immediately afterward, the entire rehab began. The pain that followed was insurmountable, leaving him fraught and anguished.
«It hurt so much to learn to stand up and walk again. I cursed the physiotherapist during that time.»
Yet, amidst the turmoil, Andy found comfort in the unwavering support of his loyal friends. They stood by his side through the darkest times, ensuring he never felt alone. Even during the joyous season of Christmas, they refused to leave his side and celebrated together, giving him hope and lifting his spirits.
After seven long weeks, Andy was allowed to return home. However, his battle was far from over. Another surgery was scheduled due to a wound healing disorder, followed by two high-dose chemotherapies coupled with the extraction and processing of stem cells from his body.
Chemotherapy Complications
The first therapy worked well for Andy. He was mentally strong, and his body responded positively. But, the second high-dose chemotherapy was less smooth–different departments, different staff, and more complications.
Despite the setbacks, Andy’s spirit remained unyielding, even when the medical professionals told him he had a mere four years to live. He thought, «Now more than ever!»
«You feel miserable during high-dose chemotherapy. The body changes, and everything hurts.»
His determination grew stronger, and he embraced life with renewed vigor. As the year drew to a close, on December 11, 2019, Andy’s unwavering faith paid off, and his goal of not spending another Christmas in the hospital came to fruition. Andy was finally allowed to go home.
Learning to Live a New Life
Andy’s life has transformed significantly from what it used to be in the past. He now receives therapy every two weeks and can no longer work in his usual job.
On the positive side, his body has adapted to regular therapy, and although he experiences numbness in his fingers and toes, there are no other side effects.
However, coping with these circumstances is undeniably challenging. «Getting through the long days and staying positive is not always easy, especially when you used to be so active in the past. What’s interesting is that you can’t see what I am going through on the outside. That can be both a good and a bad thing because it means nobody sees the struggles I face internally.» However, amidst the struggles, Andy remains grateful because he knows his situation could have been much worse. «I could have ended up in a wheel- chair or even died.»
Relying on Mental Health Trainer
Initially, Andy relied on a mental health trainer to support his emotional well-being. ”She took away my fear of things I couldn’t control. During the high-dose chemotherapy, she helped me calm down by shifting my focus from the negative to the positive. Thanks to her, I have learned not to question everything, not to have too high expectations of myself, and not to worry about the future.”
Now, Andy finds solace in the unwavering support of his girlfriend, who lost her former partner to cancer. “She went through something similar and understands me. Mutual understanding is the most important thing to make ends meet together. Because when someone has cancer, the whole family has cancer.”
Empathetically sharing his advice with others facing similar challenges, Andy emphasizes that every person’s story is unique, but how we deal with it matters. He says that mental training can make a significant difference in coping with difficult situations.
Despite his journey, Andy maintains a positive outlook. As far as multiple myeloma is concerned, he understands that advancements in medicine are constantly being made, which gives him hope for the future.

«Whether there will be enough time for me is written in the stars. But I have great friends and a wonderful partner I will marry soon. I am very grateful for all these positive vibes.»
Photo courtesy of Andreas Wicki
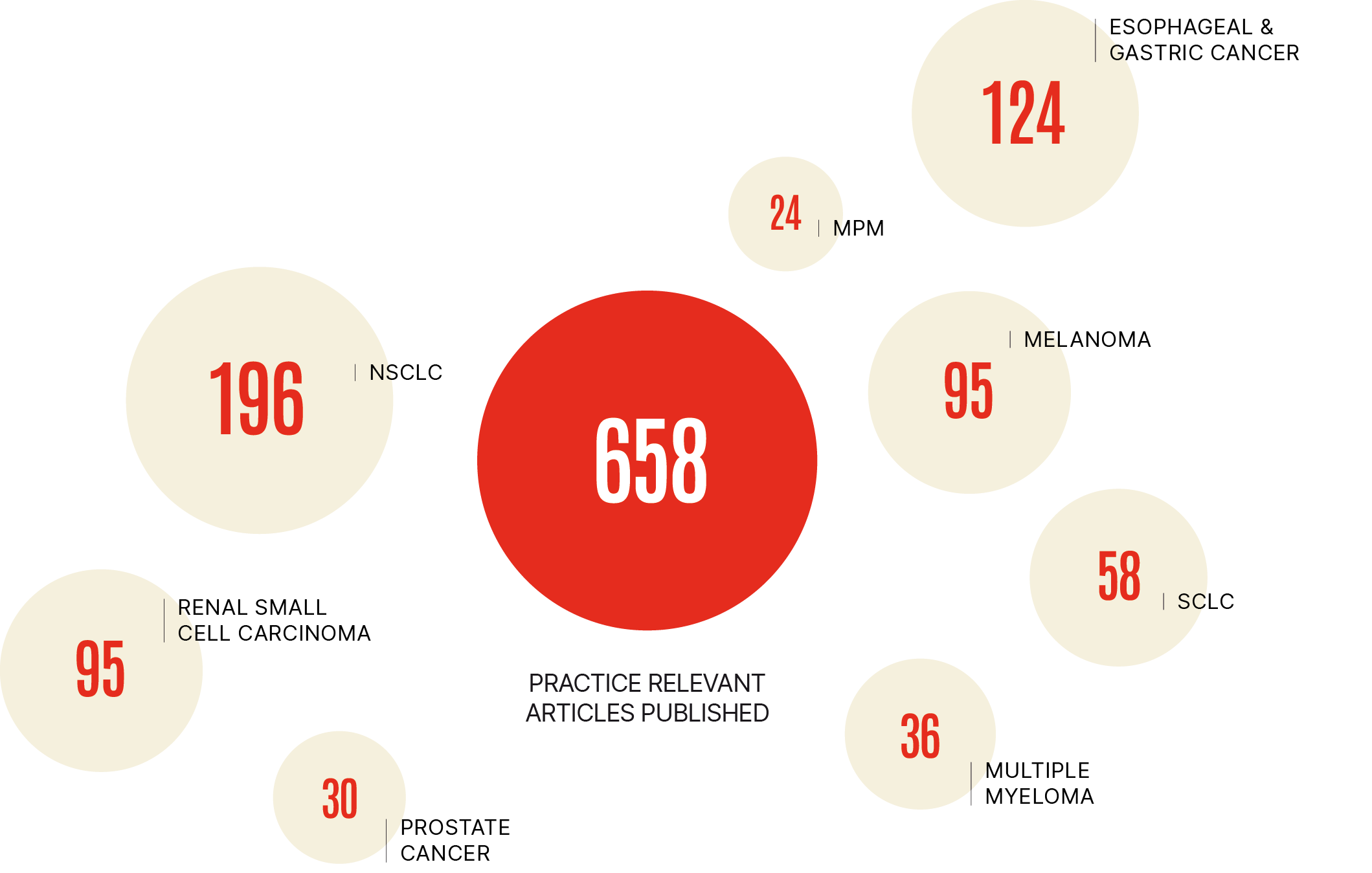
GROWING NUMBER OF PRACTICE CHANGING PUBLICATIONS
Filter 33 highly relevant studies on Multiple MyeloMa
INTERVIEW WITH DR. DAVID J. CHUNG
WHAT LIES AHEAD FOR AUTOLOGOUS STEM-CELL TRANSPLANTATION?

The majority of patients are not cured with their initial treatment. Dr. David J. Chung believes that by exploring different combinations of newer therapies, a higher fraction of cures might be achieved.
Photo: Dr. David J. Chung
High-dose chemotherapy (HDT) with autologous stem cell rescue, aka Autologous Stem-Cell Transplantation (ASCT), has been the standard of care for Multiple Myeloma (MM) patients since the 1990s. However, the therapeutic arsenal is rapidly evolving. Digest asked Dr. David J. Chung, a renowned expert in this field: Will it continue to remain an integral part of treating MM?
In such a scenario, is it possible that ASCT will go obsolete in the near future? We discussed this with David J. Chung, Director of Adult Bone Marrow Transplant and Cancer Immunotherapy Fellowships at Memorial Sloan Kettering Cancer Center (MSKCC), New York City, to learn more about his perspective on ASCT’s role in the battle against MM.
Dr. David J. Chung, the latest additions of treatments, like immunomodulatory agents and proteasome inhibitors, are pushing the boundaries of treatment options and offering new hope to patients. What are your thoughts on these new agents in development?
I think the critical aspect when approaching myeloma therapy is that we’re fortunate that there are a lot of new treatments available. Myeloma, over the last decade, has witnessed significant advances with the introduction of new classes of drugs, whereas prior to that, the treatment options were fairly limited. With the newer agents, the goal is to try and optimize their usage and figure out the best combinations, sequencing, and timing of different therapies.
The overriding principle when approaching someone with myeloma is that you want to optimize the treatment to get the best response possible during each phase of treatment. I say each phase of treatment because, unfortunately, myeloma is technically not curable with all the available treatments. There’s probably a small fraction of people who, after finishing their initial treatment, are cured, but it’s such a small fraction that we generally don’t talk about a cure for myeloma. So, with these new treatments, we’re striving to get closer to a cure.
«There is a growing preference for a four-drug combination.»
Getting back to what I was saying, when a patient goes through any phase of treatment, we’re trying to optimize the treatment to get the best response possible in order to get the best duration of control. This is essential because myeloma is not a single disease. It’s very heterogeneous, and a one size fits all approach doesn’t work, especially concerning patients with standard risk versus high-risk disease.
That being said, do you think ASCT still has a place in MM treatment, given the introduction of novel agents like monoclonal antibodies?
Previously, the standard of care for newly diagnosed patients was the triplet regimen therapy–three drugs, usually an IMiD, a proteasome inhibitor, and dexamethasone. But now, with the advent of CD38 monoclonal antibodies, specifically daratumumab, a relatively new- er drug, there is a growing preference for a four-drug combination as the initial therapy. The reason for that, again, is to maximize response while keeping in mind that you have to balance disease response with toxicities or side effects from the therapies.
Now, when it comes to ASCT, the general approach to any newly diagnosed myeloma patient, in most cases, is still to do stem cell collection for the purpose of a possible transplant. But the field is evolving, and people have re-addressed the issue of transplants and where it fits in terms of timing. In general, the clinical trial data still support the role of transplant in the up-front setting, at least as of now.
Our approach at MSKCC is to offer transplants to patients when they’re newly diagnosed if they are transplant eligible. Even if the patients are hesitant to proceed with a transplant, we still recommend stem cell collection so they can at least have that as an option in the future.
Is there a reason why you recommend transplants to patients?
The reason why we generally recommend transplant is, again, to optimize therapy for the best, deepest, and longest possible response. And clinical trials still support that as a general strategy. There are two large studies that you may already be aware of.
First, the IFM 2009 study comparing RVD alone versus RVD with a transplant showed a significant progression-free survival (PFS) benefit of about a year in patients who received a transplant. Second, the Determination Study that was more recently published found that the PFS of patients who had a transplant was more than 20 months, better than the patients who did not receive a transplant. There are more details to that, but in general, that’s the rationale for why we still recommend it. As we progress, I think several issues will ultimately influence how transplant continues to be a part of myeloma therapy.
«As of today, transplant remains the standard care.»
Can you give an example of one such issue?
For instance, the role of assessing response to therapy. Currently, the best tool at our disposal is the Minimal Residual Disease (MRD) testing that we do at various stages of treatment to assess the depth of response. There’s a clear signal that the deeper the response, meaning those who achieve MRD negativity, do better in terms of PFS and overall survival (OS). Nonetheless, how we can use that to guide treatment decisions is evolving.
At present, no studies have been published that show you can use MRD testing at a single time point to make a decision. Sustained MRD negativity is critical. And again, that speaks more to the issue of really trying to get the deepest response possible, as that may trans- late to a longer period of MRD negativity, which, again, is more important than a one-time measure.
«We don’t want an absolute age cutoff for transplants.»
Where oncology is moving now, specifically in myeloma, newer immune-based treatments have been approved in the relapse setting. But as with all new treatments for myeloma, they are now being explored in earlier lines
of therapy and ultimately will be explored as first-line or front-line therapy.
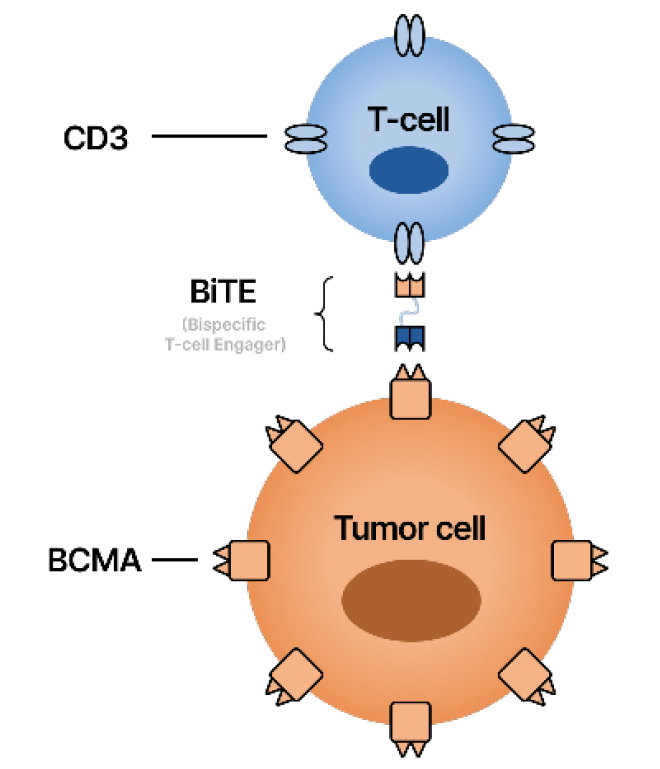
I’m specifically talking about Chimeric Antigen Receptor (CAR) T-cell therapies and bispecific antibody treatments. Both treatments leverage the immune system to exert an anti-tumor effect, and it’s quite possible that several years from now, if we were to have the same conversation, we will be talking about those as the front-line treatments and then transplant at some point might be moved to later-line therapy. But as of today, transplant remains the standard of care for MM, at least at MSKCC.
What are the decision-making factors for ASCT? Or, how do you determine if a patient with multiple myeloma is a good candidate for a stem cell transplant?
In general, myeloma patients tend to be older. The average age of diagnosis is a little around 69 years old or so–that’s an older population.
People tend to acquire various medical conditions
as they age, and there are several points to consider when deciding whether or not someone is eligible for a transplant. The first point is evaluating if a candidate is extremely elderly or very frail. Typically those are the patients who do not proceed to transplant.
However, we don’t have an absolute age cutoff for transplants. In the past, some centers had age cutoffs of 65 or 70, where anyone over those ages was not deemed eligible for a transplant. But now we realize physiological age is more important than chronological age. Meaning there are some people who are, for instance, 70 years old, but they have excellent functional status–they don’t have many medical conditions and are more akin to someone who’s 50.
On the other hand, some people are maybe 50, but have a lot of medical conditions, are physiologically older, and might be more prone to having side effects if they go through with a transplant. So it’s the overall health that we evaluate, which includes an individual’s performance status, as well as medical conditions.
Talking about medical conditions, there aren’t too many medical conditions that immediately exclude someone from eligibility, but that’s something we reassess over time. At a baseline, before someone has a transplant, we check their cardiac function and pulmonary status, as well as their renal and hepatic function.
I would say that, in my experience, there are very few people who can’t get through a transplant, even if they have pre-existing medical conditions requiring input from a cardiologist or a pulmonologist. What we tell people is that if you have more medical conditions at a baseline, you’re at a higher risk of having more toxicity from the transplant, which could translate into having a longer recovery from the treatment.
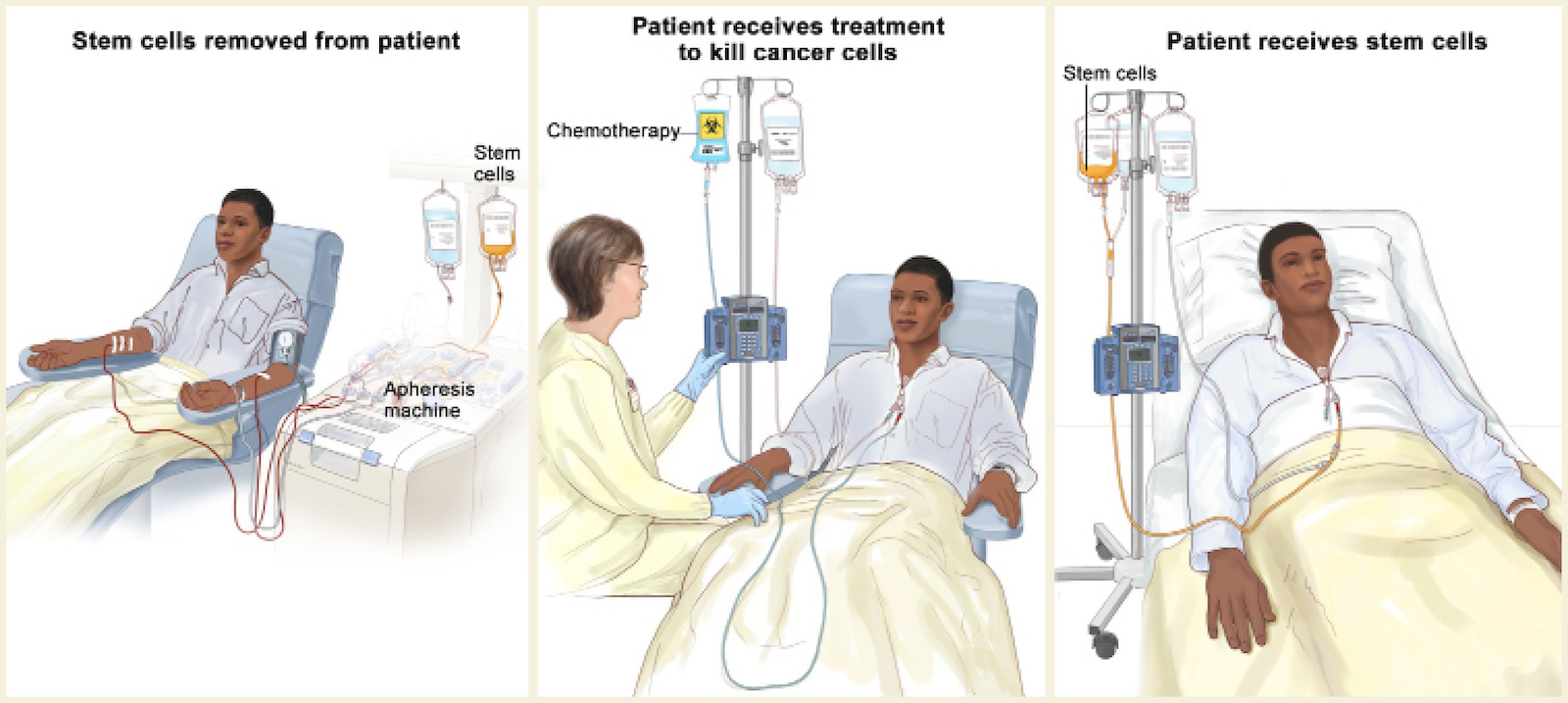
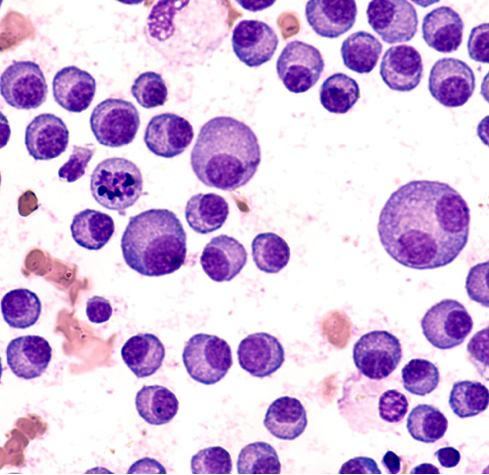
The complete blood count (CBC) is a test that measures the levels of red cells, white cells, and platelets in the blood. If there are too many myeloma cells in the bone marrow, some of these blood cell levels can be low.
Photo: © 2021 Terese Winslow LLC
What is the best sequence of treatment for newly diagnosed patients?
The current phases of treatment are induction treatment, which is the initial treatment that someone gets when they’re first diagnosed. At MSKCC, the standard is to do four drug-based or quadruplet regimens, which can include an anti-CD38 monoclonal antibody, an IMiD, a proteasome inhibitor, and dexamethasone.
We generally give four to six cycles of treatment, at which point we collect stem cells. Then if a patient is transplant eligible, we currently recommend a trans- plant, which is then followed by maintenance treatment, typically Revlimid. We continue Revlimid maintenance for as long as someone responds well and their myeloma remains under control.
«We are using the MRD test to evaluate the stem cells we collect for transplant.»
Now, if we speak in five years, it might be that for certain patients, we might say the best upfront treatment might be a CAR T-cell or something else. But currently, our standard is what I just described in terms of induction treatment, consolidation with the transplant, and then maintenance therapy.
Are there any trial results you anticipate coming out this year that might inform ASCT for MM? Or, are there any new developments or advancements in ASCT that you think will improve outcomes for patients with multiple myeloma?
I can’t think of any study off the top of my head that will result within the next year. But we’ve done a study here where we have examined the role of the MRD status
of patients’ stem cells. The MRD test is done on bone marrow samples to see how deep their responses are to each phase of treatment.
We’ve adopted that and are using this test to evaluate the stem cells we collect for transplant. We reported these results at the ASH 2022 annual meeting. It was an oral presentation where we showed that patients who undergo an ASCT with an MRD-negative stem cell graft have superior PFS and OS compared to patients with an MRD-positive stem cell product.
Ultimately, we believe that might be one way to stratify people and make treatment decisions after transplant, where if you see that a patient got a transplant with an MRD-positive graft, that person is at risk of relapsing earlier. So you might want to modify what you do after the transplant beyond standard maintenance therapy.
Another thing we know from our internal data is that patients who receive quadruplet induction therapy (the CD38 antibody with an IMiD, a proteasome inhibitor, and dexamethasone) tend to have very high rates of MRD-negative stem cells.
Is there a particular area of unmet need that should constitute the key focus for ASCT research efforts in 2023?
I think it’s more of what we’ve already covered, specifically which patients need a transplant upfront versus later. And is transplant going to continue to be the standard of care after initial therapy, or will it be supplanted by another treatment like a CAR T-cell, a bispecific, or combinations?
Currently, the vast majority of people with myeloma are not cured with their initial treatment. But several years from now, as we explore different combinations of newer therapies, might we achieve a higher fraction of cures? That’s something that’s evolving and will have to play out over time.
How does the future of MM, and particularly ASCT, look like?
Research efforts focusing on the biology and immuno-biology of myeloma will be important in honing treatments and the sequencing of treatments, both with and without a transplant. As we gain more insights into myeloma disease biology, it will allow us to optimize therapeutic approaches and ultimately improve clinical outcomes.

Memorial Sloan Kettering Cancer Center at 1275 York Avenue in New York.
Photo: MSKCC
NEW YORK, UNITED STATES
Memorial Sloan Kettering Cancer Center
The Memorial Sloan Kettering Cancer Center’s (MSKCC) mission is to end cancer for life. The personalized and expert care offered to patients of all ages at the center is driven by research from the Sloan Kettering Institute. Scientists across MSK collaborate to conduct innovative research that informs their understanding of cancer and enhances their ability to prevent, diagnose, and treat it.
Alongside being one of the world’s renowned cancer centers, MSKCC has been recognized as one of the top two cancer hospitals in the country by U.S. News & World Report for over 30 years.